
|
Current Projects |
|
Tissue Engineering a Pancreatic Substitute Based on Autologous Cells
The development of a living biological substitute for treatment of diabetes is hampered by the low availability of donor tissue and/or the need to immunosuppress the transplant recipient. Tissue substitutes based on non-pancreatic cells retrieved from the same patient and engineered for physiologically responsive insulin secretion have the potential to overcome both of these limitations. The overall goal of this research program is to produce the fundamental knowledge and enabling technologies for developing an efficacious and immune acceptable tissue substitute based on potentially autologous non-beta cells. We aim to engineer a substitute consisting of two components: one comprised of recombinant hepatic cells and the second of recombinant enteroendocrine cells. We hypothesize that the first cell type will produce the slower, more sustained secretory response, and the second the more acute phase of insulin secretion, so that, in combination, the two cell types will better mimic the function of normal beta cells. Kiranmai Durvasula, Stephanie Duncanson and Aubrey Tiernan are working on this project. Collaborators include Professor Peter Thule, VA Hospital and Emory University, Atlanta, GA; and Professor Nicholas Simpson, Department of Medicine University of Florida, Gainesville, FL.
Confocal laser-scanning microscopy of the engineered human enteroendocrine NCI-H716 cells. Views of the same transduced, differentiated NCI-H716 cells expressing insulin–EGFP fusion protein (green fluorescence) and immunostained for GLP-1 (red fluorescence). Co-localization of insulin–EGFP fusion protein with GLP-1 is demonstrated by the green fluorescence occupying the same compartments as the red fluorescence [Tang, S.C. and A. Sambanis, Biochem Biophys Res Commun, 2003. 303: 645-52].
Cryopreservation of Tissue Engineered Substitutes
To realize its potential, tissue engineering must generate substitutes that can be preserved in the long-term. Preservation is essential for off-the-shelf availability, storage and distribution of constructs fabricated at a large-scale at centralized locations, as well as for sterility testing and quality control. With autologous cell therapies, preservation allows for the later use of cells and tissue constructs by the same patient. The overall goal of this research is to understand how the various cryopreservation parameters affect cellular and tissue construct function, and on the basis of this knowledge to develop rational, systematic and quantitative preservation protocols. We are evaluating the effect of ice-forming and ice-free cryopreservation (vitrification) on the cells and biomaterial of a model pancreatic tissue substitute consisting of insulin-secreting cells encapsulated within a hydrogel. Specifically, our studies 1) determine cell osmotic tolerance limits and cryoprotectant cytotoxicity and define the domain of conditions under which to cryopreserve cells used in tissue substitutes; 2) characterize in vitro the effect of cryopreservation on construct structure and function; 3) evaluate the in vivo functionality of cryopreserved substitutes. The effect of cryopreservation on cells is assessed through cell viability, apoptosis, and metabolic and secretory functions, the former evaluated by 13C labeling and isotopomeric analysis and the latter by basal and induced insulin secretion assays; and on biomaterials through their structural integrity and functionality. Students working on this project include Hajira Ahmad, Alison Stucky and Chun Yong. Collaborators include Dr. Ying Song, Medical College of Georgia, Augusta, GA; Professor Nicholas Simpson, Department of Medicine University of Florida, Gainesville, FL; and Dr. Kelvin Brockbank, Cell and Tissue Systems, Inc.
Non-Invasive Monitoring of Tissue Engineered Implants
Non-invasive monitoring of tissue substitutes provides a very important link between construct implantation and end-point physiologic effects. We have opted to use nuclear magnetic resonance (NMR) imaging and spectroscopy methods to assess the structural integrity and functionality of tissue constructs in vitro and in vivo. Previous work focused on using 1H NMR spectroscopy in vitro and in vivo, and 31P and 13C NMR spectroscopy in vitro to assess aspects of construct function. More recently, we are focusing on monitoring the dissolved oxygen (DO) concentration in tissue implants, as oxygen availability is a critical parameter that directly influences construct function and DO can be measured by 19F NMR spectroscopy. For this, we are co-encapsulating a perfluorocarbon (PFC) emulsion with the cells and are using the correlation between the T1 relaxation of 19F and the DO level to monitor the latter in the capsules. In vitro experiments using an NMR-compatible perfusion bioreactor and support circuit are being expanded to in vivo studies with a small animal model. The student working on this project is Fernie Goh. Our lab collaborates with Dr. Robert C. Long, Jr., Department of Radiology, Emory University; and with Professor Nicholas Simpson, Department of Medicine University of Florida, Gainesville, FL.
Incorporation of perfluorocarbons (PFCs, such as perfluorotributylamine and perfluoro-15-crown-5-ether) allow the monitoring of dissolved oxygen (DO) concentration in tissue implants by 19F NMR. In this application, a population of cell-free beads contains one type of PFC and is used to measure the DO concentration at the implantation site. The experimental beads contain cells and a second type of PFC.
Development of Novel Cell Encapsulation Materials
This is a new project focusing on incorporating T-cell chemorepellent in cell encapsulating hydrogels. It is hypothesized that the combination of passive and active immunoprotection afforded by size exclusion and chemorepulsion will improve the survival of cells post-implantation relative to either approach alone. Stephanie Duncanson is working on this project, and we collaborate with Dr. Mark Poznansky at Massachusetts General Hospital, Boston, MA, and with Dr. Nicolas Chronos at the St. Joseph’s Translational Research Institute, Atlanta, GA.
Schematics of passive only (left) and passive and active immunoprotection (right), the latter comprised of incorporating a chemorepellant in the encapsulation matrix.
Bioreactor Systems for Islet Culturing and Evaluation
This project focuses on developing bioreactor systems for culturing neonatal and adult porcine islets, and on developing and using perfusion bioreactors for cell characterization. Bioreactors providing a defined and controlled chemical and fluid mechanical environment may allow for easier and possibly improved functional development of islets relative to static, fed-batch cultures. Furthermore, we are developing and implementing single-pass perfusion systems for characterizing the secretory response of free and encapsulated islets and other insulin-secreting cells to evaluate their functionality in vitro and after various periods of in vivo implantation. Students working on this project include Saif Al-Mamari and David Detwiler. In this project, our lab collaborates with Drs. Collin Weber and Susan Safley, Department of Surgery, Emory University School of Medicine. |
|
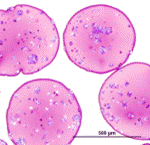
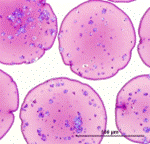
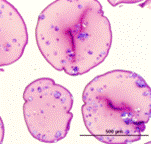
Cryosubstitution removes ice by using organic solvents, thereby allowing visualization of ice domains. This image shows cryosubstitution of frozen and vitrified alginate capsules at -90°C. Capsules subjected to conventional freezing show considerable ice formation throughout the matrix, whereas vitrified capsules show no ice domains [Mukherjee, N., et al., Cell Transplant, 2005. 14: 449-56; 2. Song, Y.C., et al., Transplant Proc, 2005. 37: 253-5]. |
|
Fresh Vitrified |
|
Conventionally frozen |
|
Histology of fresh, vitrified and conventionally frozen alginate capsules also indicates more damage to the matrix caused by conventional freezing. |
|
Schematic of single-pass perifusion system used to measure secretory response of free and encapsulated islets and other cells. |
